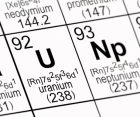
4. Does depleted uranium pose a radiation hazard?
All isotopes of uranium are radioactive. Both uranium and depleted uranium, and their immediate decay products, emit alpha and beta particles and a small amount of gamma radiation.
Depletion of U-235 during processing leaves DU appreciably less radioactive than naturally occurring isotopic mixtures. It typically contains 30-40 per cent of the concentration of U-235 found in natural uranium, or about 0.2 to 0.3 per cent by weight. This means that the radioactivity of newly produced DU is only about 60 per cent of natural uranium.
DU munitions collected in Kosovo also contained trace amounts of other radioactive elements, but they increase the overall radioactivity by less than one per cent.
All natural uranium isotopes emit alpha particles – positively charged ions identical to the nucleus of a helium atom, with two protons and two neutrons. Their beta and gamma activity is low. Alpha particles are relatively large, and do not penetrate far in tissue – they are stopped by the skin, for example. This means uranium only poses a radiation hazard if it is breathed in, eaten or drunk, or enters part of the body exposed by injury.
The GreenFacts Three-Level Structure used to communicate this SCHER Opinion is copyrighted by Cogeneris SPRL.