Electromagnetic Fields 2009 Update
1. Introduction to electromagnetic fields
- 1.1 What are electromagnetic fields?
- 1.2 How have the health risks of electromagnetic fields been reassessed?
1.1 What are electromagnetic fields?
The SCENIHR opinion states:
3.1. Introduction
The purpose of this opinion is to update the SCENIHR opinion of 21 March 2007 in the light of newly available information, and to provide a methodological framework and corresponding guidelines to evaluate available scientific evidence in order to ensure the best possible quality for risk assessment.
In order to update the opinion, this section establishes the scientific rationale which is needed to provide the requested opinion. Relevant scientific knowledge from the physical, engineering, medical and biological sciences is critically evaluated and summarised. When appropriate, gaps in knowledge are highlighted and suggestions for future important areas of research are included. This opinion also addresses the issue of children's sensitivity, and in particular dosimetry aspects of radiofrequency exposure of children.
As in the previous opinion, the section is divided into four separate sub-sections based on frequency bands (radio frequency (RF) (100 kHz < f ≤ 300 GHz), intermediate frequency (IF) (300 Hz < f ≤ 100 kHz), extremely low frequency (ELF) (0 < f ≤ 300 Hz), and static (0 Hz) (only static magnetic fields are considered in this opinion). These frequency ranges are discussed in order of decreasing frequency: RF, IF, ELF, and static fields, respectively. For each frequency range the review begins with a description of sources and exposure to the population. This is followed, for each frequency range, by a discussion that is organised according to outcome. For each outcome, relevant human, in vivo, and in vitro data are covered.
There are also frequency bands that are not covered in this opinion since relevant data regarding possible effects on human health are not available or not directly mentioned in the mandate. This includes a part of the radio frequency spectrum which is the lower frequency Terahertz (THz) radiation. Terahertz applications operate between the optical spectrum on the short wavelength side and the radio frequency fields on the longer wavelength side. Applications are mainly imaging and spectroscopy. Other parts of the electromagnetic spectrum that are not discussed include the infrared and ultraviolet frequency bands.
Source & ©: SCENIHR,
![]()
3.1 Introduction, p.13.
1.2 How have the health risks of electromagnetic fields been reassessed?
The SCENIHR opinion states:
3.2. Methods
This opinion represents an update of the earlier opinion of SCENIHR (2007). It is principally based on original scientific work published between January 2007 and December 2008 in English-language peer-reviewed scientific journals. The specific references that are cited constitute only a part of all the literature considered. The aim has been to cite only those studies that contribute significantly to the opinion. The inclusion criteria are discussed in detail in chapter 3.8 - Methodological framework.
Source & ©: SCENIHR,
![]()
3.2 Methods, p.13
3.8. Methodological framework
3.8.1. Introduction
The purpose of an opinion such as this is to provide the scientific background with respect to if exposure to electromagnetic fields (EMF) is a cause of disease or other health effects. The opinion is not a scientific review article which includes all papers that have been published on the subject. It has also to be stressed that it is not a tool for risk management, or a way to communicate opinions regarding exposure guidelines.
This section summarises the procedure of work which is the foundation of the opinion. It describes the process how the studies that have been included were identified and analysed. Furthermore, it gives information about the weighting process which is underlying the final conclusions regarding various types of EMF and health effects. The criteria that have been governing the evaluation process of the different kind of studies (epidemiological, human experimental, in vivo, in vitro) are provided. Methods for obtaining data on exposure and dosimetry are explained, as well as criteria for evaluating from the perspective of exposure assessment and dosimetry. When appropriate, typical strengths and weaknesses of various methods and techniques are described.
3.8.2. Criteria used
In the previous opinion a methodological section was added in response to comments received during the public consultation process. The purpose was:
- to explain the criteria for how studies were selected,
- to explain how the scientific evidence was synthesised into an assessment of the evidence for a causal effect of exposure to electromagnetic fields and health effects
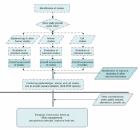
The scientific rationales for both the previous and the present opinions were developed in a way as described below (Figure 3
). As a general rule, scientific reports that are published in English language peer-reviewed scientific journals are considered primarily. Exceptions to this are specifically mentioned in the text. This does not imply that all published articles are considered to be equally valid and relevant for health risk assessment. On the contrary, a main task is to evaluate and assess the articles and the scientific weight that is to be given to each of them. Only studies that are considered relevant for the task are commented upon in the opinion. Many more reports were considered than are cited in the reference list. However, only articles that contribute significantly to the update of the opinion are cited and commented upon. In some areas where the literature is particularly scarce it has been considered important to explain why the results of certain studies do not add useful information to the database. The focus is on articles published after the presentation of the previous opinion.
The primary objectives of this health risk assessment are:
- to identify and characterise any hazardous properties (diseases, adverse health effects) of EMF in relevant biological systems
- to examine the relationship between exposure and these hazards (dose response relationship)
- to highlight the nature and extent of any uncertainties in the determination of hazards and dose response relationships
- to evaluate the plausibility of possible modes/mechanisms for each hazard of concern.
It should be emphasised that recommendations for risk management measures are excluded from the mandate of SCENIHR (see Figure 3
). Relevant research for EMF health risk assessment can be divided into broad sectors such as epidemiological studies, experimental studies in humans, experimental studies in animals, and cell culture studies. Studies on biophysical mechanisms, dosimetry, and exposure assessment are also considered. In a report of this nature it is not possible to consider the experiences of individuals. Nonetheless, such information often triggers a scientific study.
A health risk assessment evaluates the evidence within each of these sectors and then weighs the evidence across the sectors to produce a combined assessment. This combined assessment should address the question of whether or not a hazard exists i.e. if a causal relation between exposure and some adverse health effect exists. The answer to this question is not necessarily a definitive yes or no, but may express the weight of the evidence for the existence of a hazard. If such a hazard is judged to be present, the risk assessment should also address the magnitude of the effect and the shape of the dose-response function, i.e. the magnitude of the risk for various exposure levels and exposure patterns. A full risk assessment also includes exposure characterisation in the population and estimates of the impact of exposure on burden of disease.
Epidemiological and experimental studies are subject to similar treatment in the evaluation process. It is of equal importance to evaluate positive and negative studies, i.e. studies indicating that EMF has an effect and studies not indicating the existence of such an effect. In the case of positive studies the evaluation focuses on alternative explanations for the positive result: with what degree of certainty can one rule out the possibility that the observed positive result is produced by bias, e.g. confounding or selection bias, or chance. In the case of negative studies, one assesses the certainty with which it can be ruled out that the lack of an observed effect is the result of (masking) bias, e.g. because of too small exposure contrasts or too crude exposure measurements; one also has to evaluate the possibility that the lack of an observed effect is the result of chance, a possibility that is a particular problem in small studies with low statistical power. Obviously, the presence or absence of statistical significance is only one factor in this evaluation. Rather, the evaluation considers a number of characteristics of the study. Some of these characteristics are rather general, such as study size, assessment of participation rate, level of exposure, and quality of exposure assessment. Particularly important aspects are the observed strength of association and the internal consistency of the results including aspects such as dose response relation. Other characteristics are specific to the study in question and may involve dosimetry, method for assessment of biological or health endpoint, the relevance of any experimental biological model used etc. Regarding experimental studies, additional important characteristics that are taken into consideration are the types of controls that have been used and to what degree replication studies have been performed. For a further discussion of aspects of study quality, refer for example to the Preamble to the IARC Monograph Series (IARC 2006). It is worth noting that the result of this process is not an assessment that a specific study is unequivocally negative or positive or whether it is accepted or rejected. Rather, the assessment will result in a weight that is given to the findings of a study.
The step that follows the evaluation of the individual studies within a sector of research is the assessment of the overall evidence from that sector with respect to a given outcome. This implies integrating the results from all relevant individual studies into an overall assessment. This is based on the evaluations of the individual studies and takes into account, for each study, both the observed magnitude of the effect and the quality of the study. Note again, that for this process to be valid, all studies must be considered equally irrespective of their outcome.
In the final overall evaluation phase, the available evidence is integrated over various sectors of research. This phase involves combining the existing relevant pieces of evidence on a particular end-point from studies in humans, animal models, in vitro studies, and from other relevant areas. The integration of the separate lines of evidence should take place as the last, overall evaluation stage, after the critical assessment of all (relevant) available studies for particular end-points. In the first phase, epidemiological studies should be critically evaluated for quality irrespective of the putative mechanisms of biological action of a given exposure. In the final integrative stage of evaluation, however, the plausibility of the observed or hypothetical mechanism(s) of action and the evidence for that mechanism(s) is a factor to be considered. The overall result of the integrative phase of evaluation, combining the degree of evidence from across epidemiology, animal studies, in vitro and other data depends on how much weight is given on each line of evidence from different categories.
3.8.3. Specialised sections
Specific considerations that are relevant for evaluation of the studies are presented in more detail in the text below. This gives the framework for how the present opinion is developed after evaluation of specific studies. Although some of the more important aspects to consider are discussed, it must be pointed out that this is not a complete text on the subject.
3.8.3.1. Dosimetry and exposure assessment
Accurate and reliable dosimetry and exposure assessment are key requirements of scientific studies on biological effects of electromagnetic fields. It is imperative to select the adequate assessment tool to identify exposure conditions. There are several tools available, e.g. frequency selective measurement equipment, broadband probes, exposimeters or numerical methods. The adequate selection of the equipment depends on the type, the magnitude and the variability of the signals from the emitting sources and the purpose of the study. For in vitro, in vivo and human studies adequate exposure setups have to be selected to guarantee reproducible and accurate exposure of the biological samples or volunteers. Well defined exposure conditions at the site of the biological test object are an imperative requirement. Only studies including the uncertainty of such determinations are complete.
To determine exposure arising from electromagnetic fields several approaches are available. The main possibilities are measurements and calculations. When performing measurements different methods exist. One can distinguish between spot measurements, monitoring, and individual exposure assessments (Neubauer et al. 2005).
Measurements of electromagnetic fields can be performed in two ways: broadband and frequency selective. Broadband measurements give the total contribution over a wide frequency range without distinguishing the contributions of different sources operating at different frequencies. Frequency selective measurements allow these specific contributions to be identified. Broadband measurements are performed with probes and hand-held measuring instruments, while for frequency selective measurements spectrum analysers attached to antennas are used. Both frequency selective and broadband measurement equipment can be used to perform spot measurements, i.e. measurements at a given location and at a specific time. A major shortcoming of spot measurements is that they do not reflect the variation of the exposure versus time and in space. When needing to assess the exposure of individuals, spot measurements therefore have limitations.
If it is necessary to assess the exposure variations versus time at a specific location, monitoring systems are adequate solutions. Such systems allow continuous monitoring of the whole frequency range for all types of signals in the frequency range of interest. Monitoring systems do usually not reflect the exposure of moving individuals. Due to the fact that exposure is often highly variable in space, other solutions are needed to assess individual exposure of persons.
Exposimeters allow personal exposure to electromagnetic fields over time to be determined. It is crucial for the evaluation of the electromagnetic fields that one can monitor different electromagnetic sources in a way that allows distinction between the contributions from different applications, e.g. mobile phones, GSM and UMTS base stations, or broadcast stations. Exposimeters are quite promising tools, although they also have some limitations. First, they have a limited bandwidth and do not allow the exposure from all electromagnetic sources to be assessed. Moreover, they have a limited dynamic range, i.e. very weak and very strong signals cannot be assessed. In addition, they give only a surrogate of the exposure. Exposimeters are usually worn on the back or on a belt, and therefore indicate the field level close to the body. This is in fact not exactly the exposure, since exposure is defined as the electromagnetic field level at the location of the exposed person assessed without the person being present.
In addition to measurements, calculations of the exposure are often an adequate approach. In many cases the combined use of measurements and calculations is appropriate. Analytical approaches are often used to get preliminary information regarding the exposure conditions. However, they are neither suitable for describing complex exposure conditions nor to describe the field distribution inside the human body in an accurate way. For such purposes numerical tools can be used. Such numerical methods can be divided into two different main types based on the used physical wave propagation model, i.e. field theoretical methods (solving Maxwell’s equations) and optical methods. Examples of field theoretical methods are the Finite Elements Method (FEM), Finite Differences in Time Domain (FDTD) or the Methods of Moments (MOM). Examples of optical methods are ray launching and ray tracing. The literature contains also hybrid methods, which are a combination of field theoretical methods and optical methods. Field theoretical methods, e.g. FDTD, are often used for the investigation of small areas whileoptical methods like ray tracing are often applied to large areas.
The selection of an adequate exposure metric is imperative for scientific studies. As long as the biological mechanisms related to a specific exposure, e.g. low level RF exposure, are unknown, it might even turn out that several exposure metrics have to be evaluated. One approach is to focus only on the exposure above a certain threshold. Another concept is to assess cumulative exposure when a linear dose–response association without a threshold is expected. It is also possible that the exposure variability might be relevant or a mixture of the three concepts mentioned has to be applied. Moreover, differences in signal characteristics might be of relevance. One viewpoint is that physical characteristics apart from intensity are not relevant; the other is to support the idea that frequencies, modulation, and intensities might play an important role. In addition, the exposure timing might also be of relevance (Neubauer et al. 2007).
Adequate and accurate exposure assessment is crucial for the evaluation of exposure conditions of both workers and the general population. The selection of suitable measurement equipment or adequate calculation tools is an imperative requirement. Moreover, both measurements and calculations should only be performed by highly qualified personnel. When performing in situ measurements it is necessary to take environmental factors such as the impact of objects or weather conditions into account. In addition, the variability of the exposure due to environmental conditions needs to be considered. Measurement equipment has to be calibrated in regular intervals. Moreover, the variability of exposure conditions due to factors such as changes of data rates or changes in the current on power lines has to be considered. Another crucial aspect is the impact of the human body on the measurement results. Moreover, results without uncertainty analysis are incomplete.
It is imperative to take multisource exposure into account to get reliable information on exposure. If measurements or calculations are performed, aspects like whole body or localised exposure need to be considered.
Similar considerations have to be made when performing in vitro, in vivo or human studies. The exposure set up needs to allow reproducible and accurate exposure, i.e. the electromagnetic field must be well defined at the site of the cell culture or in specific tissue of animals or volunteers. The sensitivity of experimental variations on the induced fields such as posture or size of test animals should be minimised. Moreover, environmental conditions such as temperature, humidity, and background electromagnetic fields have to be controlled. Uncertainty analysis of the exposure has to be developed because it is an important part of the overall study uncertainty. The exposure set up should minimize additional stress to test objects or volunteers apart from the exposure itself. The complete exposure set up including all controlling and monitoring devices should be immune against electromagnetic interference under worst case test conditions. The list of dosimetric requirements given here is not intended to be complete; more information can be found in different publications (e.g. Valberg 1995, Kuster and Schönborn 2000, Portier 1994, Lang 2004).
In recent years a trend towards improvement of dosimetric aspects in scientific studies on biological effects of electromagnetic fields could be observed. Exposure is in most cases sufficiently reported. However, in several cases the uncertainty of the assessment is not given. In some epidemiological studies the contribution of RF sources other than mobile phones to the exposure is not taken into account. Several experimental human studies suffer from non-optimised exposure set-up, and the assessment of the SAR is often based on phantom measurements which may be more or less representative for the actual subjects. Such approaches are suitable for compliance testing, but not adequate for scientific investigations.
3.8.3.2. Epidemiology
Epidemiology is concerned with the study of the occurrence and distribution of diseases in populations. Its ultimate goal is to learn about the causes of disease that may lead to effective preventive measures. However, in contrast to experiments or clinical trials, epidemiological studies are usually observational and are therefore vulnerable to bias and confounding. Thus, criteria are needed to assess whether observed empirical exposure- disease associations are possibly causal or more likely a play of chance or methodological artefacts. Making sense of results from epidemiological studies is particularly challenging when they are conflicting, or when there is a discrepancy between epidemiological and experimental findings.
The range of observational study types reaches from rather simple descriptive studies to analytical studies. In evidence-based decision making, different observational study types contribute with different weights. More confidence relies on results derived from well- conducted prospective cohort studies than on results from case-control studies, whereas firm conclusions are rarely drawn from cross-sectional studies and particularly descriptive studies. Nevertheless, there is a considerable range of quality within study types. This applies especially to case-control studies which are the most commonly used in investigations of hazards of chronic diseases like cancer. Case-control studies are prone to selection bias and recall bias and a transparent description of the study material and procedures is a necessary requirement to evaluate the study’s quality.
Criteria to be discussed when summarising the overall epidemiological evidence are temporality and strength of the observed association, a convincing dose-response pattern, internal and external consistency of results, the specificity of the association, and the absence of bias and confounding. Importantly, reported relative risk estimates have to be compatible with the absolute effects observed in the disease rates over time.
Meta-analyses are a useful tool to numerically summarise the evidence, but if substantial heterogeneity is identified, a structured approach trying to clarify the source of such heterogeneity is more important than the calculation of pooled estimates. A good meta- analysis or review can be seen as a study of studies; hence, like original studies, they vary considerably in quality. The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement is an important guideline on how to report results of observational studies and is promoted by many influential journals as a new standard (e.g. von Elm et al. 2007).
3.8.3.3. Human laboratory studies
Experimental studies in laboratory or other controlled settings are used to evaluate whether effects can be observed during or shortly after exposure to a causal (risk) factor. These studies are also called provocation studies, i.e. the study will try to answer the question whether a certain exposure will trigger (provoke) a certain effect, e.g. a physiological reaction or symptoms. The quality of experimental studies on humans may vary. It is of utmost importance that the design and protocol of the study are described in detail by the authors reporting the results of the study. If this is not the case, it may be impossible to judge if the results are valid or not.
Laboratory studies, as compared to epidemiological studies, have the advantage of providing better possibilities to control the exposure factor(s) under study, as well as possible confounding factors. On the other hand, the relevance of experimental laboratory studies to the real life situation may be less clear. For example, the absence in laboratory settings of contributing factors present in everyday life may influence the results and possibly reduce the chance to discover an effect.
A double blind experimental laboratory study where subjects are randomly allocated to two or more exposure conditions is considered the strongest design to study acute effects. The goal is to have contrasting exposure conditions but otherwise as similar conditions (and groups) as possible to compare in the analyses of possible effects. Subjects should be randomly allocated to the different exposure conditions. A cross-over design, where the same individuals are exposed to both (or several) conditions in a random order, is preferred. In the cross-over design, the subjects serve as their own controls in the comparisons between e.g. sham and the exposure under study. If two separate groups of participants are assigned either to sham or the exposure under study, other possible differences between the groups than the exposure conditions may influence the results. The cross-over design may however be biased by carry-over effects if the time between the two (or more) conditions are not long enough for possible effects to wash out. If that is the case, a true effect may be hidden. Effects due to the order in which exposure conditions are applied may also obscure the results if the numbers of subjects that begin with the separate conditions are not balanced. For example, unfamiliar routines and environment may produce different reactions during the first experiment as compared to the later sessions. Habitation sessions during which the participants will be acquainted with the procedures and setups may be useful to avoid this problem. In order to prevent expectations of participants or researchers to distort the results it is important that the study is performed double blinded, i.e. neither the researchers that lead the experiments nor the participants are aware of the true state of the exposure conditions during the study.
The choice of study group will have an impact on the external validity of the study, i.e. which populations the results are valid for. A very homogenous group (e.g. with regard to age and gender or symptom profile) may limit the population that the results may be generalised to. On the other hand, a more heterogeneous study group may risk missing an effect present only in one or several sub-groups.
The outcomes that are assessed in a study may be more or less robust. If possible, objectively measured (e.g. heart rate, blood chemistry etc) data are desired. Self- reported effects are more difficult to assess. The choice of scales for self reported effects or interpretations of open questions may also have significant influence on the results.
3.8.3.4. In vivo
Animal studies are frequently based on experiments using laboratory strains of mice or rats. The advantage of animal studies is that they provide information about effects on a whole living organism that displays the full repertoire of body structures and functions, such as nervous system, endocrine system and immune responses. In this respect, animal studies are usually a more powerful experimental tool than cellular studies for assessing health risks to humans. However, extrapolation to humans is not straightforward since there are obvious differences in e.g. body mass, life expectancy, physiology, and metabolism between species. Rodent carcinogenicity studies, for example, have been criticised because many agents that are carcinogenic in rodents (often only at very high doses) are not carcinogenic to humans, and some human carcinogens do not affect rodents in standard carcinogenicity tests (Ames and Gold 1990, Trosko and Upham 2005, Anisimov et al. 2005). Extrapolation from animal experiments to humans should always include consideration of the validity of the animal model used – good animal models do not exist at present for all human diseases. Nevertheless, at a molecular level, many basic processes, such as DNA damage and repair, are similar in animals and humans, and animal studies have remained a cornerstone in evaluating toxicity of chemical and physical agents. In the evaluations of IARC, for example, agents for which there is sufficient evidence of carcinogenicity in animals are considered to pose carcinogenic hazard to humans, unless there is scientific evidence that the agent causes cancer through a species-specific mechanism that does not operate in humans (IARC 2006).
Criteria for evaluating individual animal studies include the following questions: (i) Was the number of animals per group adequate? (ii) Were animals of both sexes used (if relevant)? (iii) Were animals randomly allocated to groups? (iv) Were exposure levels and treatment durations appropriate? (v) Was the duration of observation adequate with respect to the health endpoint addressed (for example, lifetime observation in carcinogenicity studies)? (vi) Apart from the exposure of interest, was treatment of exposed and control groups identical? (vii) Was there possibility of bias related to differences in survival between groups? (viii) Was the endpoint measured adequately? (ix) Was data reported adequately? (x) Was a dose-response relationship observed? (xi) Were the exposure system and dosimetry adequate?
These criteria are valid for any animal study, but quality of exposure system and dosimetry is particularly important in studies on RF fields. In such studies, the exposure system is often a compromise between restraint-related stress and the accuracy of RF dosimetry. If animals are allowed to move freely during RF exposure, they change their position and orientation in relation to the electromagnetic wave and may also be shielded by other animals, which results in large uncertainties in dosimetry. Therefore, immobilisation of animals has been used in many animal studies to achieve well-defined dosimetry. However, immobilisation can cause restraint-related stress that might affect the outcome of the experiment (no experimental bias is caused if both exposed and the sham-exposed animals are restrained, but stress could act as an effect modifier and obscure possible RF-induced effects). Such effects of stress can be reduced by appropriate steps, such as the habituation of animals to restraint.
3.8.3.5. In vitro
In vitro studies are used to investigate toxicological, mechanistic, and other relevant effects which can provide evidence for and possible understanding of the development of cancer and other diseases. In vitro assays can show potential effects of various agents on a wide variety of biological endpoints in a manner which is rapid and cost-effective. The role for in vitro assays in hazard identification is thus obvious.
Genotoxic studies include assays showing the interaction of the possible risk factor with the DNA. Non-genotoxic studies often aim to give mechanistic understanding by using a wide variety of endpoints. This can elucidate the machinery of action on the cellular level which can also be predictive to a certain extent for some hazardous effects. It has to be pointed out that in vitro studies contribute to acute toxicity testing and can provide information regarding tumorigenesis, and other physiological or pathological processes, but it cannot replace in vivo conditions or long term exposure conditions. Therefore information about genotoxic capacity for example, can only be indicative of a potentially serious public health risk.
For evaluation of published data, criteria are needed to distinguish between useful and not useful studies for the assessment. In general, for toxicological studies it is imperative to set up the accurate experimental control samples. Positive and negative controls within in vitro studies provide evidence for controlled experimental conditions. In EMF research it is preferable to use sham exposure as a control condition as well, and performing experiments in a blinded manner.
Exposure has to be performed under fully controlled conditions regarding field exposure (frequency conditions, flux density, SAR-values etc.), temperature, CO2 etc. and has to be documented. Furthermore, a proper dosimetry has to be presented.
For risk evaluation, studies of dose dependency are needed to determine possible threshold values.
It is evident that the appropriate cell types have to be used for specific experimental approaches for proper identification of biological effect.
Concerning statistical power, both the number of parallel samples during the experiment and the number of independent replicates of an experiment have to be considered.
To provide information about genotoxic capacity, a battery of techniques and methods are available, ideally, the used methods should confirm and/or compensate each other. Therefore, it is necessary to prove positive findings by using different techniques (Table 2
). In addition, the reproducibility of positive findings has to be shown by independent laboratories. For non-genotoxic studies the same criteria mentioned above are valid. In vitro studies are very helpful when they are producing specific and reproducible results, however, the biological relevance can be unclear and the extrapolation of data is rather difficult. An isolated finding should not be overestimated; it has furthermore to be proven by independent laboratories. For risk assessment it is useful to consider functional studies that are investigating several cellular processes (and/or alterations in physiological processes). The novel methods that on a large scale (by high-throughput screening; “-omics”) can study e.g. gene transcription, protein expression and modification, and cellular metabolism can be instrumental in elucidating possible mechanistic cellular action of an agent.
Table 2. Advantages and disadvantages with certain commonly
used cyto-/genotoxic assays.![]()
Source & ©: SCENIHR,
![]()
3.8 Methodological framework, p. 50-59
The Three-Level Structure used to communicate this SCENIHR Opinion is copyrighted by GreenFacts asbl/vzw.